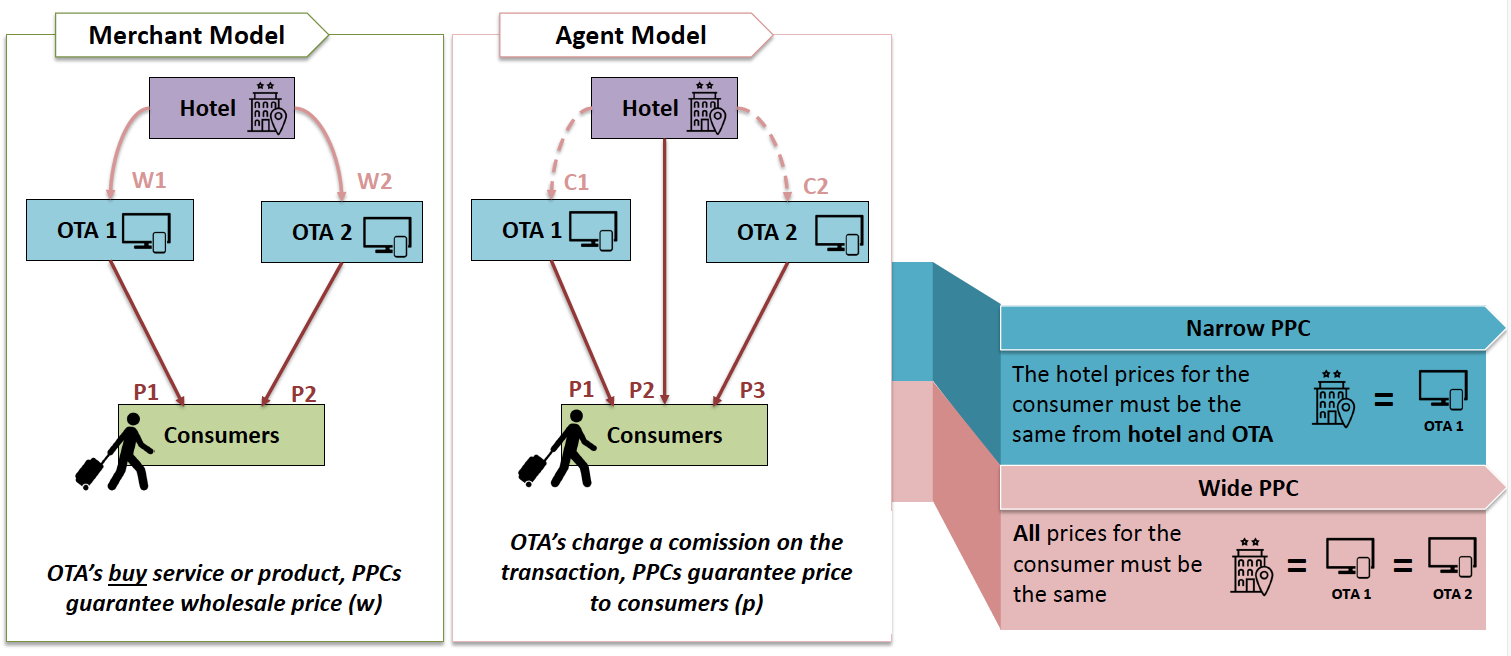
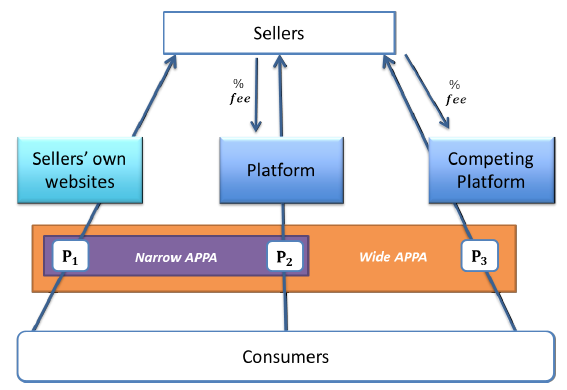
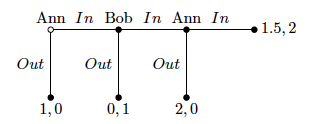
This is the first in a series of posts highlighting competition issues and cases that are set to drive the debate in Europe this year.
Pfizer and Flynn Pharma: a major decision from the CMA
On 7 December 2016, the United Kingdom’s (UK’s) Competition and Markets Authority (CMA) issued a potentially precedent-setting decision against pharmaceutical producer Pfizer and distributor Flynn Pharma, imposing a fine of nearly £90 million for excessive pricing.
In September 2012, Pfizer sold the distribution rights to its anti-epilepsy drug Epanutin (phenytoin sodium) to Flynn Pharma, which debranded (or “genericised”) the drug, with the effect that it was no longer subject to price regulation. Following the sale, Pfizer increased its price for phenytoin sodium to Flynn Pharma by between 780% and 1,600% relative to the price at which it had previously sold the drug in the UK, and in turn Flynn Pharma increased the wholesale price of the drug to between 2,300% and 2,600% of the former price.
A key feature of phenytoin sodium appears to be that patients taking the drug cannot readily switch to the same drug manufactured by another producer, since even minor differences in production processes could affect the efficacy of the drug in treating epilepsy in individual patients. Therefore, despite the fact that the drug was genericised, the CMA appears to have found that Pfizer and Flynn Pharma retained a de facto monopoly over the sale of the drug to existing patients taking Epanutin. Such a finding would also imply that alternative epilepsy treatments were not viable substitutes for phenytoin sodium in respect of the relevant patients, and were therefore not included in the definition of the relevant market.
The excessive pricing debate
The prohibition of excessive or unfair pricing by dominant firms is a controversial part of UK and European competition law (it has no meaningful counterpart in US legislation or case law). On the one hand, there are strong economic arguments, at least from a static point of view, for preventing a dominant firm from exploiting its monopoly position by charging prices higher than the theoretical competitive price. One of the key results of microeconomic theory (and indeed the foundation of competition policy) is that monopoly pricing lowers overall welfare compared to a competitive market outcome, since the monopoly maximises profits by producing a less-than-efficient quantity of the relevant good and selling it at a higher-than-efficient price.
However, enforcing a prohibition against excessive pricing presents various difficulties. One of these is to establish a benchmark price against which the actual price charged by the dominant firm is to be evaluated, and deciding whether the margin above this benchmark is excessive. According to the CMA press release, it appears to have had regard both to the initial regulated price of phenytoin sodium, and the price charged by Pfizer in other European countries, in reaching a finding of excessive pricing.
It is important to note, however, that there is no inherent reason why such prices should represent useful comparators. In other words, although a price increase of 2,600% naturally appears alarming at first glance, a range of factors could have resulted in the initial price being very low, especially if it was regulated. In this case, Pfizer and Flynn Pharma argue that the regulated price of Epanutin in the UK prior to September 2012 had been loss-making. It remains to be seen how the CMA established a relevant benchmark when its non-confidential decision is made public.
A further risk in enforcing a prohibition of excessive pricing, partly related to the issue discussed above, is that it could have a negative impact on firms’ dynamic incentives to invest across the economy. For example, over-enforcement could prevent a firm from earning economic profits where it has innovated in order to gain a temporary competitive advantage. More generally, over-enforcement runs the risk of creating uncertainty, and thereby lowering incentives to invest, if businesses fear that their future profits will be capped by a competition authority at a level which they cannot predict in advance.
For such reasons, economists such as Massimo Motta and Jorge Padilla (both teaching in the Competition masters at BGSE) have proposed that excessive pricing provisions should be enforced only in cases where there is little or no prospect of the relevant market eventually correcting itself, and where a sector regulator would not be better placed than the competition authority to intervene (among further restrictive conditions). In this case the CMA may have concluded that the inability of other phenytoin sodium producers to compete for existing Epanutin patients created such a situation where entry is infeasible. Even so, the question remains whether this issue could not better be addressed through amending existing drug price regulation. The release of the CMA’s final decision is likely shed more light on this issue.
What to look out for in 2017
In the meantime, Flynn Pharma has appealed the CMA’s decision to the Competition Appeals Tribunal (CAT). 2017 could therefore reveal how the CAT views the different considerations surrounding excessive pricing, and to what extent the CMA decision will be applicable to other drugs and industries. The finding of excessive pricing also raises the prospect that Flynn Pharma’s customers, and specifically the UK Department of Health, could sue it for damages resulting from the high price, which would raise further interesting issues in so-called “private” excessive pricing enforcement.
References
Evans, D.S. & Padilla, A.J. 2005. “Excessive Prices: Using Economics to Define Administrable Legal Rules”. Journal of Competition Law and Economics 1(1), pp. 97–122.
Motta, M & de Streel, A. 2007. “Excessive Pricing in Competition Law: Never say Never?” The Pros and Cons of High Prices, pp. 14-46. Swedish Competition Authority.